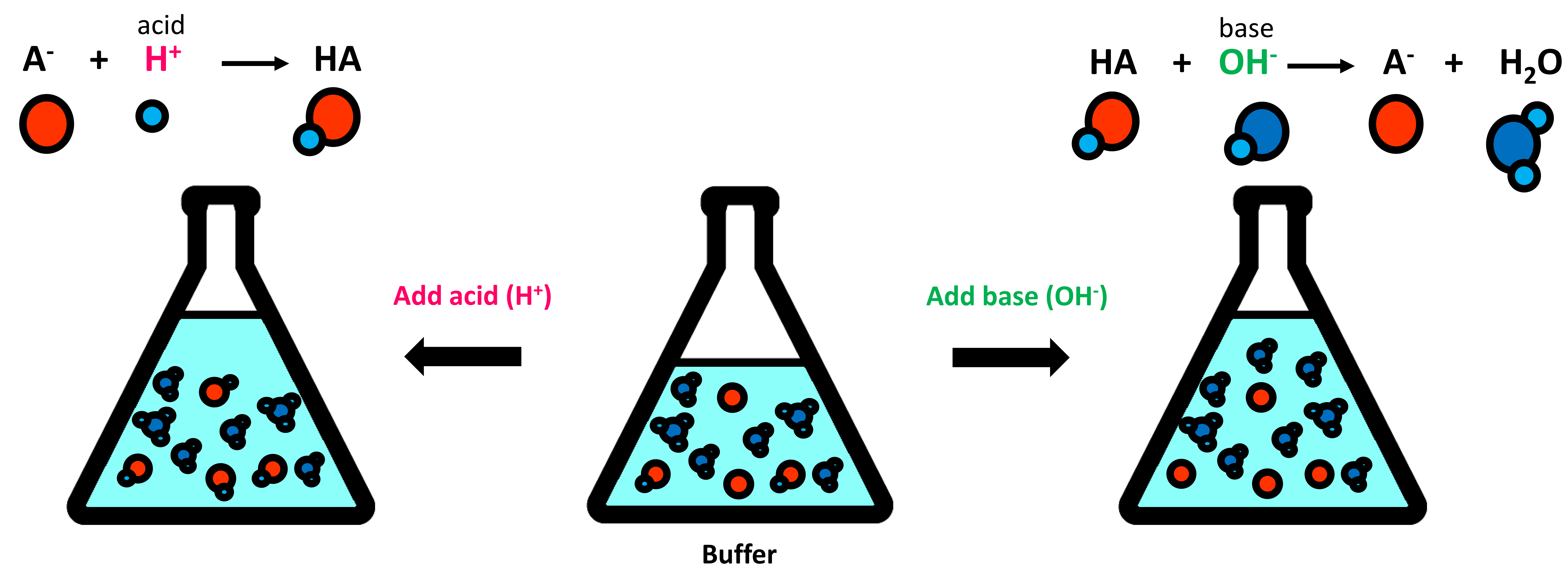
What Is A Buffer How Does It Form . a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is a buffer composed of? a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. How does a buffer work? Selecting proper components for desired ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: the mechanism involves a buffer, a solution that resists dramatic changes in ph.
from goldbio.com
the mechanism involves a buffer, a solution that resists dramatic changes in ph. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. How does a buffer work? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Selecting proper components for desired ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph.
What is a Biological Buffer and How to Choose the Best Buffer for Your
What Is A Buffer How Does It Form buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. Selecting proper components for desired ph. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. How does a buffer work? Buffers do so by being composed of certain pairs of solutes: what is a buffer composed of? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer How Does It Form buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. Buffers do so by being composed of certain pairs of solutes: what is a buffer composed of? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Is A Buffer How Does It Form.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is A Buffer How Does It Form How does a buffer work? the mechanism involves a buffer, a solution that resists dramatic changes in ph. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Selecting proper components for desired ph. Buffers do so by being composed of certain pairs of. What Is A Buffer How Does It Form.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is A Buffer How Does It Form the mechanism involves a buffer, a solution that resists dramatic changes in ph. what is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a buffer is a. What Is A Buffer How Does It Form.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory What Is A Buffer How Does It Form a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers do so by being composed of certain pairs of solutes: Selecting proper components for desired ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a solution whose ph is. What Is A Buffer How Does It Form.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is A Buffer How Does It Form the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: Selecting proper components for desired. What Is A Buffer How Does It Form.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Is A Buffer How Does It Form How does a buffer work? Buffers do so by being composed of certain pairs of solutes: a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a solution whose ph is not altered to any great extent by the addition of small quantities. What Is A Buffer How Does It Form.
From www.youtube.com
More About Buffers (A2 Chemistry) YouTube What Is A Buffer How Does It Form a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Selecting proper components for desired ph. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. buffer, in chemistry, solution. What Is A Buffer How Does It Form.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is A Buffer How Does It Form a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. what is a buffer composed of? the mechanism involves a buffer, a solution that resists dramatic changes in ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph.. What Is A Buffer How Does It Form.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID5083921 What Is A Buffer How Does It Form a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. the mechanism involves a buffer, a solution that resists dramatic changes in ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. what is a buffer composed. What Is A Buffer How Does It Form.
From app.jove.com
Buffers, Buffer Components and Buffer Action Concept Chemistry JoVe What Is A Buffer How Does It Form a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. the mechanism involves a buffer, a solution that resists dramatic changes. What Is A Buffer How Does It Form.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer How Does It Form the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Selecting proper components for desired ph. Buffers do so by being composed of certain pairs of solutes: How does a. What Is A Buffer How Does It Form.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your What Is A Buffer How Does It Form what is a buffer composed of? How does a buffer work? Selecting proper components for desired ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a. What Is A Buffer How Does It Form.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital What Is A Buffer How Does It Form Buffers do so by being composed of certain pairs of solutes: Selecting proper components for desired ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the mechanism involves a buffer,. What Is A Buffer How Does It Form.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists What Is A Buffer How Does It Form a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. what is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: How does a buffer work? a solution whose ph is not altered to any great. What Is A Buffer How Does It Form.
From www.slideserve.com
PPT Buffering Capacity Addition of STRONG Acids or Bases PowerPoint What Is A Buffer How Does It Form a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. How does a buffer work? a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. the mechanism involves. What Is A Buffer How Does It Form.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk What Is A Buffer How Does It Form a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Buffers do so by being composed of certain pairs of solutes: a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or. What Is A Buffer How Does It Form.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Buffer How Does It Form a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer solution is a solution where the ph does not. What Is A Buffer How Does It Form.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Buffer How Does It Form a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. How does a buffer work? the mechanism involves a buffer, a. What Is A Buffer How Does It Form.